Immuneering Announces Extraordinary 86% Overall Survival at 9 Months in First-Line Pancreatic Cancer Patients Treated with Atebimetinib + mGnP
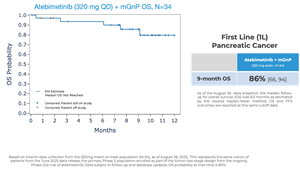
- 86% overall survival (OS) observed at 9 months; standard of care benchmark is ~47% -
- 53% progression-free survival (PFS) observed at 9 months; standard of care benchmark is ~29% -
- Company expects regulatory feedback on pivotal trial plans in Q4 2025 and, subject to that feedback, expects to initiate pivotal trial by the end of 2025 and begin dosing patients by mid-2026 -
NEW YORK, Sept. 24, 2025 (GLOBE NEWSWIRE) -- Immuneering Corporation (Nasdaq: IMRX), a clinical-stage oncology company focused on keeping cancer patients alive, today announced positive updated survival and safety data from its ongoing Phase 2a trial of atebimetinib (IMM-1-104) in combination with modified gemcitabine/nab-paclitaxel (mGnP) in first-line pancreatic cancer patients (N=34), with 9 months median follow up. The data, to be presented at the Pancreatic Cancer Action Network (PanCAN) Scientific Summit 2025, marks a milestone for the Company in the treatment of one of the deadliest and most treatment-resistant solid tumors. The Company also announced it will not be hosting its previously scheduled conference call.
“Overall survival is the gold standard in oncology and has been Immuneering’s goal from the very beginning. In cancer nothing matters more than keeping patients alive and helping them thrive. We are beyond thrilled to report that not only was our extraordinary 94% overall survival at 6 months sustained with additional follow up time, but that our observed 9-month overall survival of 86% shows an even larger gap with standard of care benchmarks,” said Ben Zeskind, Ph.D., CEO of Immuneering. “To combine such meaningful overall survival with such favorable tolerability has the potential to be truly game-changing for first-line pancreatic cancer patients.”
Extraordinary and Growing Survival Advantage Observed
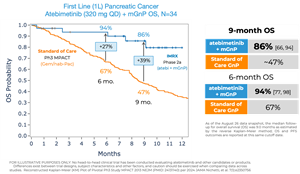
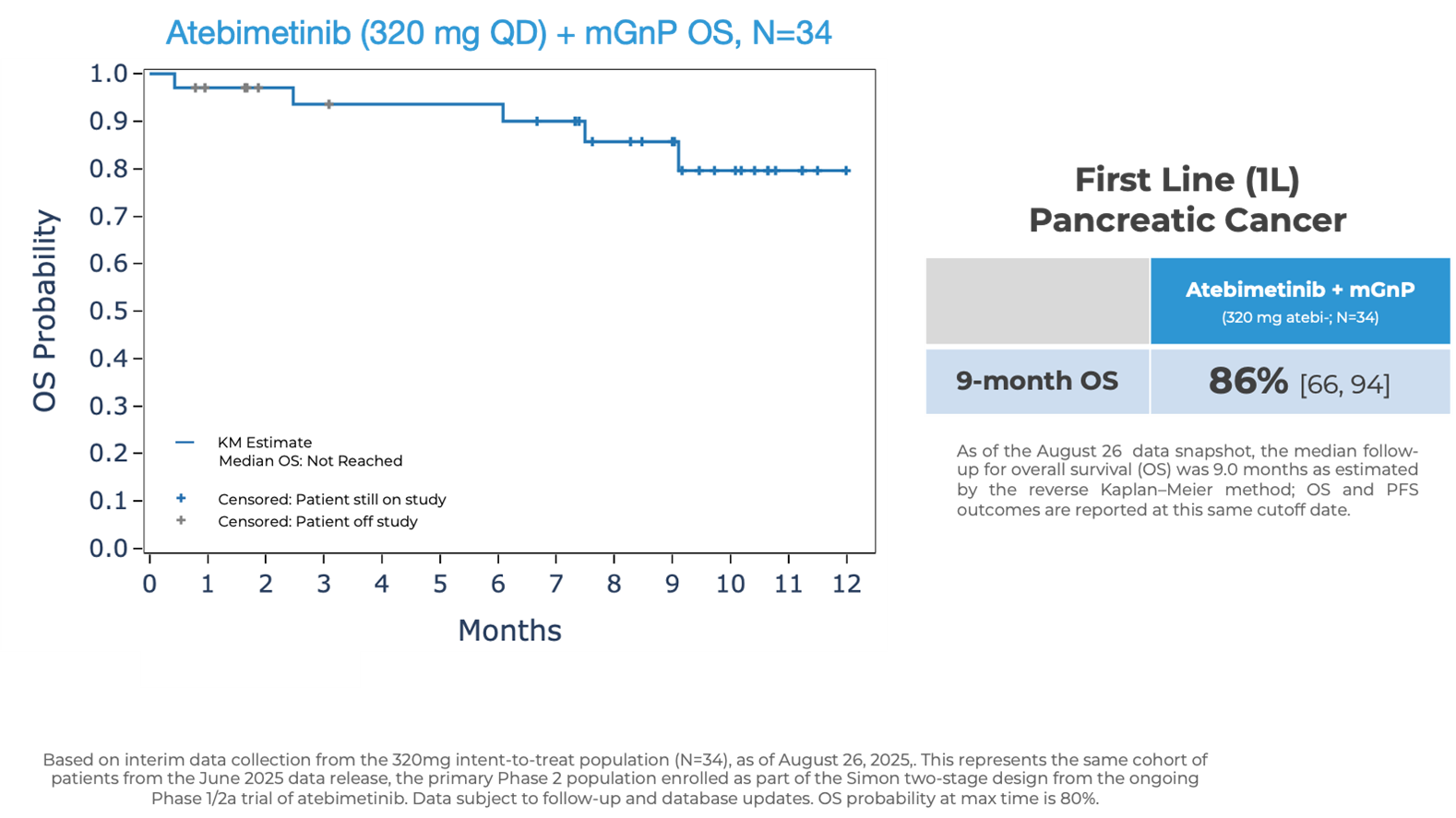
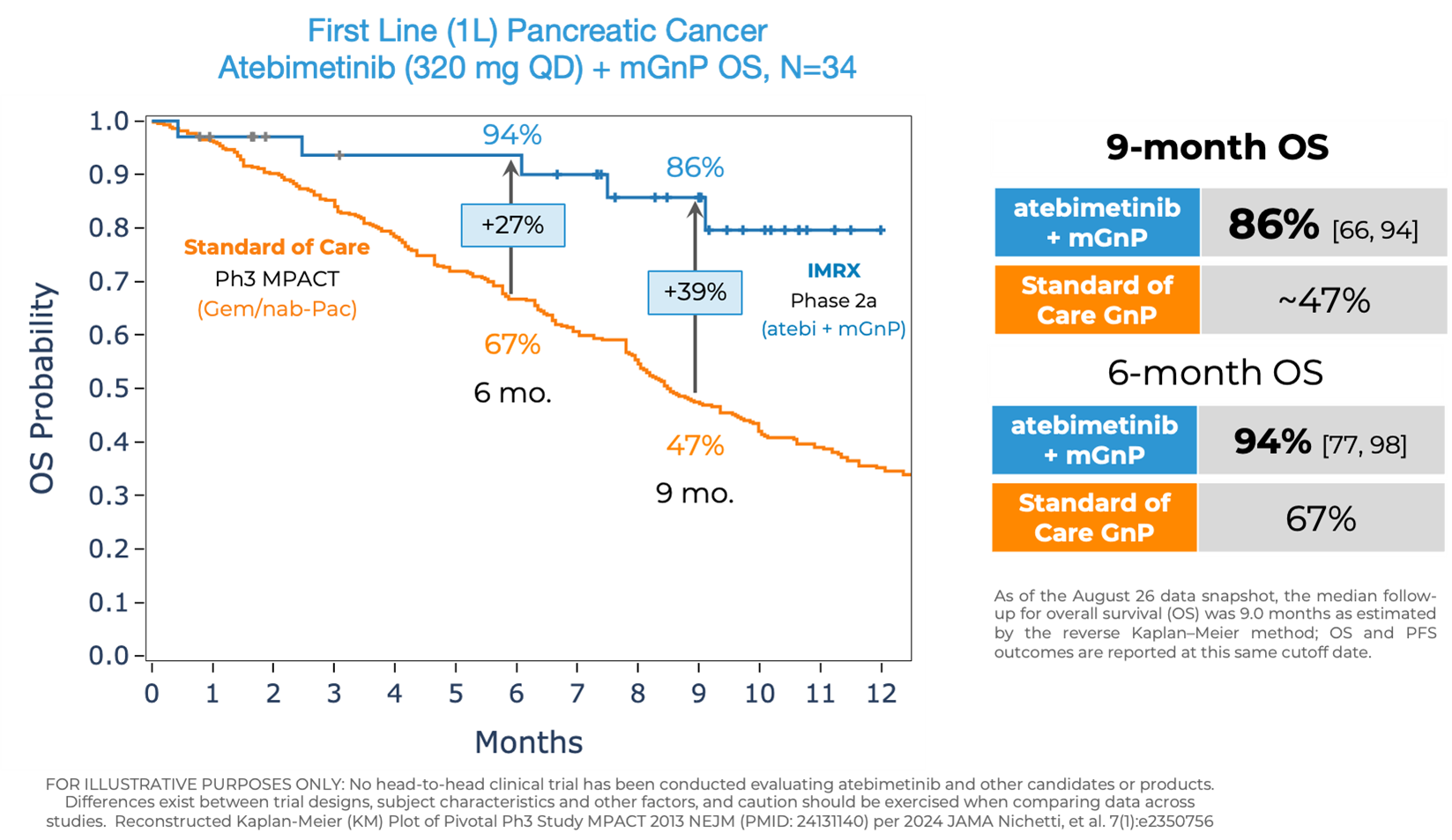
Atebimetinib (320mg dosed once-daily) + mGnP demonstrated remarkable overall survival (OS) and progression-free survival (PFS) at 9 months median follow up in first-line pancreatic cancer patients (N=34). The MPACT pivotal trial for the standard of care, gemcitabine/nab-paclitaxel, reported significantly lower OS and PFS at 9 months.
-
OS observed at 9 months was 86% in patients receiving atebimetinib + mGnP. The median OS was not yet reached as of the data cutoff date. The standard of care reported a ~47% OS at 9 months.
- As previously reported, OS observed at 6 months was 94% in patients receiving atebimetinib + mGnP. The standard of care reported a 67% OS at 6 months.
-
PFS observed at 9 months was 53% in patients receiving atebimetinib + mGnP. The standard of care reported a ~29% PFS at 9 months.
- PFS observed at 6 months was 70% in patients receiving atebimetinib + mGnP. The standard of care reported a ~44% PFS at 6 months.
- Unless otherwise specified, all data are reported using a data cutoff date of August 26, 2025, from the same patient cohort (N=34) as previously reported in June 2025. The estimates of (and other references to) standard of care with respect to the nine-month follow-up data were extrapolated and reconstructed by the Company based on the publicly available third-party MPACT pivotal trial data for gemcitabine/nab-paclitaxel. The estimates of (and other references to) standard of care set forth above with respect to the six-month follow-up data were reported out directly from the publicly available third-party MPACT pivotal trial data for gemcitabine/nab-paclitaxel. The Company’s Phase 1/2a clinical trial of atebimetinib does not include a head-to-head comparison against any other agents, and caution should be exercised when comparing data across trials.
The Company believes these compelling updated survival data reflect the potential for a durable, compounding benefit with atebimetinib + mGnP.
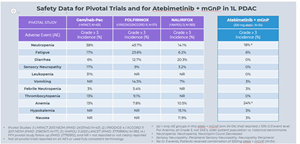
Favorable Tolerability Profile Observed:
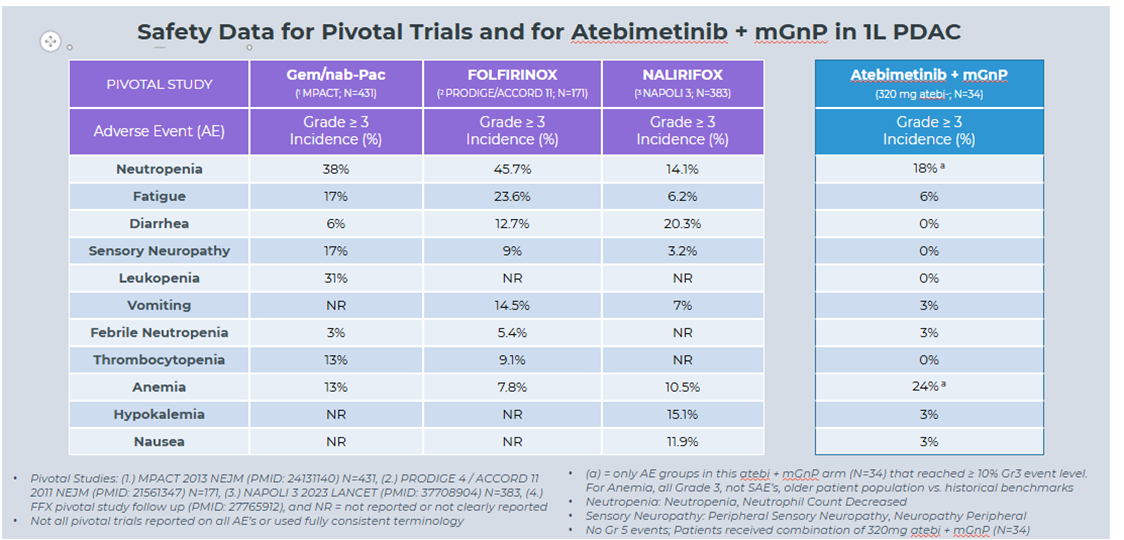
- As of the data cutoff date, Atebimetinib (320mg dosed once-daily) + mGnP continued to demonstrate a favorable tolerability profile in first-line pancreatic cancer patients (N=34), with only two categories of adverse events observed at the Grade 3 level in more than 10% of patients (neutropenia and anemia, both of which are categories commonly observed with standard of care chemotherapy). No new safety signals were identified.
How Did Atebimetinib Achieve Such Extraordinary Survival?
Atebimetinib is a Deep Cyclic Inhibitor: A New Paradigm in Targeted Therapy
- Immuneering’s proprietary Deep Cyclic Inhibitors (DCIs) challenge the conventional model of sustained or continuous inhibition in oncology.
- Most therapies are designed for sustained inhibition, driving cancer to adapt and develop resistance so tumors shrink quickly but temporarily.
- Deep Cyclic Inhibitors are designed to pulse faster than tumors can adapt, so tumors shrink slowly but durably.
- Sustained inhibition also causes suppressed transient signaling in healthy cells, leading to many adverse events.
- Deep Cyclic Inhibitors aim to restore full transient signaling to healthy cells, with the goal of leading to fewer adverse events.
Atebimetinib Targets MEK: A Broader, Potentially More Durable Approach
- MEK is a key control point in the MAPK pathway (RAS-RAF-MEK-ERK), which is pathologically activated in a majority of cancers, including approximately 97% of pancreatic cancers.
- Targeting MEK blocks a broader range of MAPK pathway alterations because it is further downstream, creating the potential for more durable benefit.
“Deep Cyclic Inhibitors like atebimetinib represent a fundamental shift in targeted therapy, away from continuous inhibition and toward pulsatile modulation of key oncogenic pathways,” said Brett Hall, Ph.D., Chief Scientific Officer at Immuneering. “This approach has the potential to deliver both durability and tolerability, two patient-centered essentials oncology has long struggled to balance.”
“Pancreatic cancer remains one of the most challenging cancers we face in the clinic with far too few treatment options available to patients and survival rates that have remained unacceptably low for decades,” said Vincent Chung, M.D., F.A.C.P., Professor, Department of Medical Oncology and Therapeutics Research at City of Hope, principal investigator of the Phase 2a clinical trial and a paid member of the company’s scientific advisory board. “I have seen firsthand in my own patients the benefits of atebimetinib’s durability and tolerability. The remarkable overall survival, progression-free survival, and tolerability data we are seeing with atebimetinib + mGnP in first-line pancreatic cancer patients, now out to 9 months of median follow up, represent an important step towards creating urgently needed new options for these patients. We are also planning a confirmatory study.”
Near-Term Milestone Expectations
Immuneering is planning for several near-term anticipated milestones related to atebimetinib, including:
- Regulatory feedback on pivotal trial plans in Q4 2025
- Announcing further updated OS and PFS data from first-line pancreatic cancer patients (N=34) treated with atebimetinib + mGnP, including at a scientific conference in 2026
- Pending regulatory feedback, initiating a pivotal Phase 3 trial of atebimetinib in combination with mGnP in first-line pancreatic cancer by the end of 2025, and dosing the first patient by mid-2026
- Initiating additional atebimetinib clinical trial combination arms in 2026, including in non-small cell lung cancer
About Immuneering
Immuneering is a clinical-stage oncology company focused on keeping cancer patients alive. The Company is developing an entirely new category of cancer medicines, Deep Cyclic Inhibitors. Immuneering’s lead product candidate, atebimetinib, is an oral, once-daily Deep Cyclic Inhibitor of MEK designed to improve durability and tolerability, and expand indications to include MAPK pathway-driven tumors such as most pancreatic cancers. Atebimetinib is currently in a Phase 2a trial in patients with advanced solid tumors including pancreatic cancer. The Company’s development pipeline also includes early-stage programs. For more information, please visit www.immuneering.com.
Forward-Looking Statements
This press release contains forward-looking statements, including within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding: our plans to develop, manufacture and commercialize our product candidates; the treatment potential of Deep Cyclic Inhibitors, including atebimetinib, alone or in combination with other agents, including modified Gemcitabine/nab-paclitaxel (mGnP), as well as the extraordinary and growing survival advantage observed and potential for a durable, compounding benefit from pulsatile modulation of signaling pathways; the timing of regulatory feedback, initiation of a pivotal trial and related patient dosing; the ability of targeting MEK to deliver a more durable benefit; the timing and content of future data releases and presentations; and the timing for the initiation of additional atebimetinib clinical trial combination arms, including in non-small cell lung cancer.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the risks inherent in oncology drug research and development, including target discovery, target validation, lead compound identification, and lead compound optimization; we have incurred significant losses, are not currently profitable and may never become profitable; our projected cash runway; our need for additional funding and ability to continue as a going concern; our unproven approach to therapeutic intervention; our ability to address regulatory questions and the uncertainties relating to regulatory filings, reviews and approvals; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in or failure to obtain regulatory approvals; our reliance on third parties and collaborators to conduct our clinical trials, manufacture our product candidates, and develop and commercialize our product candidates, if approved; failure to compete successfully against other drug companies; protection of our proprietary technology and the confidentiality of our trade secrets; potential lawsuits for, or claims of, infringement of third-party intellectual property or challenges to the ownership of our intellectual property; our patents being found invalid or unenforceable; costs and resources of operating as a public company; and unfavorable or no analyst research or reports.
These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the period ended June 30, 2025, and our other reports filed with the U.S. Securities and Exchange Commission, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, except as required by law, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
Media Contact:
Carson Creehan
817-412-1096
carson.creehan@padillaco.com
Investor Contact:
Laurence Watts
619-916-7620
laurence@newstreetir.com
Figures accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/6da3aac4-f0f7-4153-9bb9-e5f646790db8
https://www.globenewswire.com/NewsRoom/AttachmentNg/168aba3d-8079-49a8-9959-cfe8c9ec6dae
https://www.globenewswire.com/NewsRoom/AttachmentNg/80dd1ddd-4e2b-463b-ad9e-4f1361008281

Atebimetinib (320 mg QD) + mGnP OS, N=34
Atebimetinib (320 mg QD) + mGnP OS, N=34
First Line (1L) Pancreatic Cancer; Atebimetinib (320 mg QD) + mGnP OS, N=34
First Line (1L) Pancreatic Cancer; Atebimetinib (320 mg QD) + mGnP OS, N=34
Safety Data for Pivotal Trials and for Atebimetinib + mGnP in 1L PDAC
Safety Data for Pivotal Trials and for Atebimetinib + mGnP in 1L PDAC
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.